THE BASICS OF CORROSION
Corrosion is a definitive part of a metal’s lifecycle. Three factors contribute to corrosion in metals – (a) the presence of two dissimilar metals (b) an electrolyte or an environment and (c) a metallic conducting path between the dissimilar metals. Aided by environmental factors, bacteria colonise on metals. Bacterial colonization on metal substrates further promote corrosion. Additionally, pollutants accelerate corrosion and corrosion products, mainly rust. Other critical issues that aid corrosion impacting infrastructure and industry are the interrelated phenomena of climate change, global warming and greenhouse emissions.
Corrosion is a crucial worldwide problem that strongly affects natural and industrial environments. Corrosion and pollution together pollute water bodies and impair the quality of the environment. They bring down the efficiency of the industry and the durability of infrastructure assets. Corrosion is high-risk, expensive damage to everything from vehicles to industrial equipment, shipping vessels, bridges, pipelines, buildings and so on.
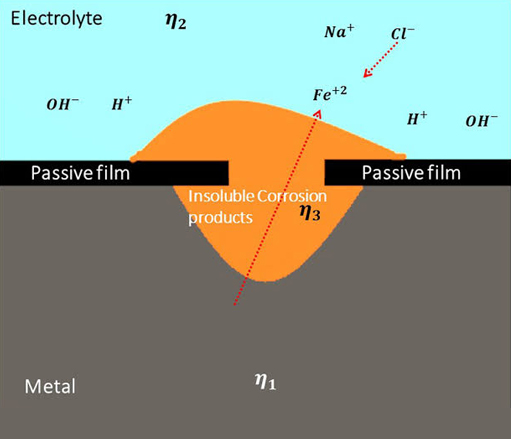
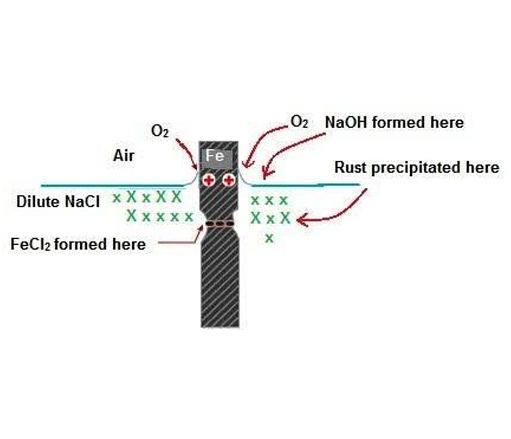
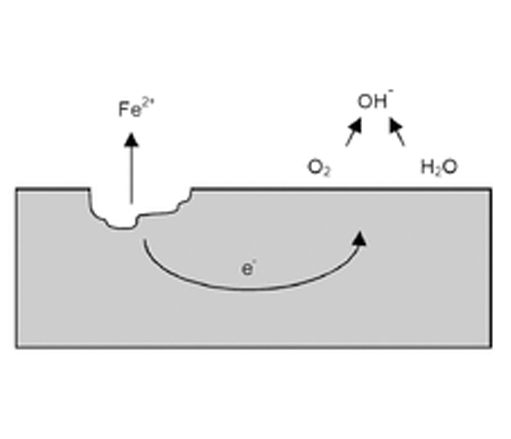
Corrosion of metals may be general material loss with discoloration and scaling, formation of deep gouges, uncontrolled enlargement of cracks and crevices or, pinhole formations. Rust is the most common appearance of corrosion. Rust is formed when metal reacts with oxygen and water, the process speeded up by salt resulting in iron (II/III) oxide (rust). Corrosion is an interdisciplinary science involving metallurgy, electrochemistry and, chemical thermodynamics. The electrochemical process of rust may be written down as:
2Fe + O2 🡪 2FeO
Iron + Oxygen 🡪 Iron (II) oxide
FeO + O2 🡪 Fe2O3
Iron (II) oxide + Oxygen 🡪 Iron(III) oxide (RUST)
In corrosion, metals lose electrons (oxidation) resulting in free ions/radicals.
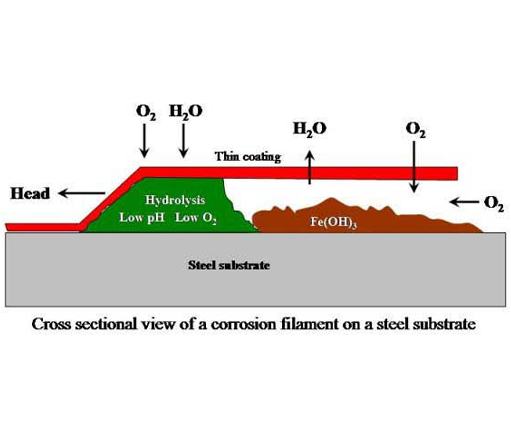
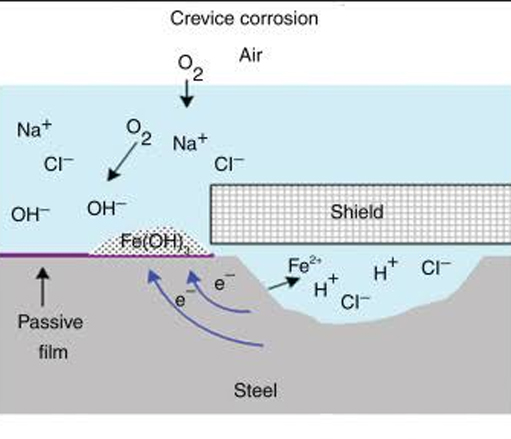
Filiform Corrosion
Occurs on metallic surfaces coated with a thin organic film that is typically 0.1 mm thick

Erosion Corrosion
Usually found at high flow rates around tube blockages, tube inlet ends, or in pump impellers
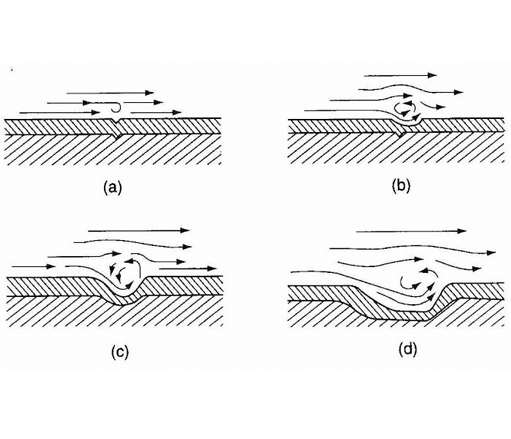
Turbulent-flow Corrosion
Acceleration in the rates of corrosion attack in metal due to continuous Turbulent Flow
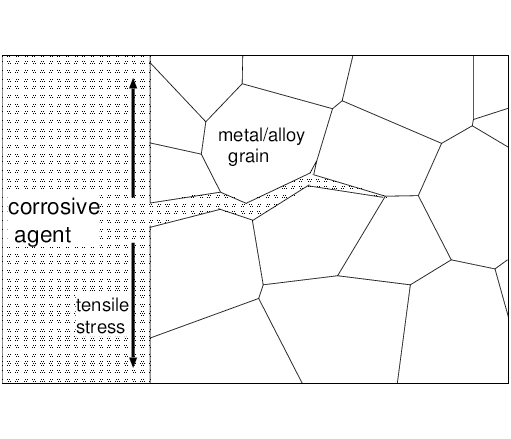
Intergranular Corrosion
Localized attack along the grain boundaries while the bulk of the grains remain largely unaffected